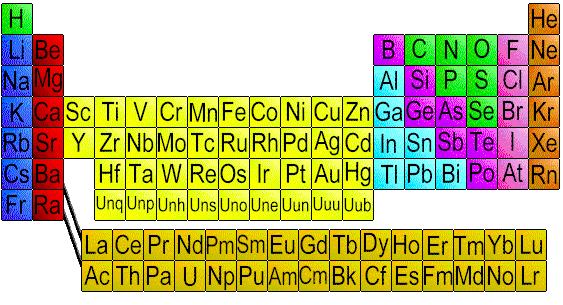


The periodic law is most commonly expressed in chemistry in the form of a periodic table, based on Mendeleyev's table, with subsequent emendations and additions, is still in widespread use. In this table the elements are arranged in seven horizontal rows, called periods, in order of increasing atomic weights, and in nine vertical columns, called the groups. The first period, containing two elements, hydrogen, and helium, and the next two periods each containing eight elements are called short periods. The remaining periods called long periods, contain eighteen elements, as in periods four and five, or thirty two elements as in period six. The long period seven includes the actinide series, which has been filled in by the synthesis of radioactive nuclei through element 103, lawrencium. Heavier transufanium elements have also been synthesized.
Period Table History IN REAL AUDIO!!
Basic Overview of Periodic Table
Periodic Table 1
Periodic Table 2
Periodic Table 3
Periodic Table 4
![]()
List of Periodic Tables
Periodic Table Database
More Periodic Table Data